- Carbon cycle: process which carbon moves from inorganic to organic compounds and back

- Photosynthesis converts inorganic carbon dioxide to organic compounds
- Consumers obtain organic compounds by eating producers
- Cellular respiration returns carbon dioxide to atmosphere
- Photosynthetic organisms produce 160 billion metric tons per year
- Carbon dioxiode makes sugars in photosynthesis
- Cellular respiration gives off carbon dioxide as waste
- Carbon dioxide in atmosphere traps heat
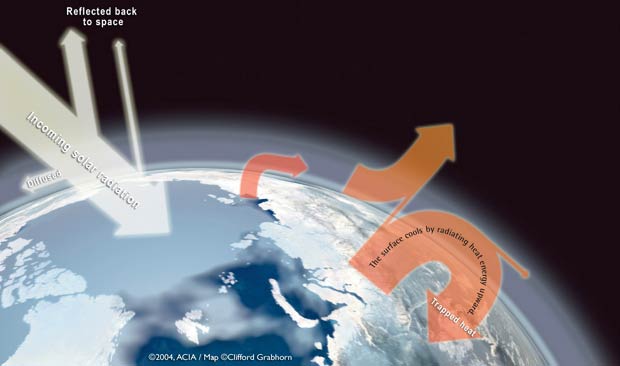
- This keeps climate warm for living things
- Atmospheric carbon dioxide has been rising
Thursday, November 13, 2008
Chapter 8.4
Tuesday, November 11, 2008
Chapter 8.3
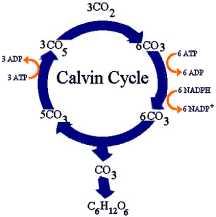
- Calvin cycle produces sugar and other organic compounds used as fuel
- Starting material is regenerated each cycle
- Starting material is RuBP, a sugar with five carbons
- Inputs of cycle: carbon dioxide, ATP, and NADPH
- Outputs: small energy-rich sugar molecule called G3P
- G3P: Raw material to make glucose and other organic molecules
- Light reactions take place in thylakoid membranes
- Convert light energy to chemical energy of ATP and NADPH
- Light reactions use water in equation and produce oxygen
- Calvin cycle takes place in stroma
- Uses ATP and NADPH to convert carbon dioxide into sugar
- Photosynhesis is first step in flow of energy through ecosystem
- Chemical energy passed from producers to consumers
- Photosynthesis is ultimate source of food and oxygen
Concept Check 8.3
1) What are the inputs and outputs of the Calvin cycle?
The inputs are carbon dioxide, ATP, and NADPH. The output is G3P.
2) Which stage of photosynthesis uses each reactant from the overall photosynthesis equation? Which stage generates each product from the overall photosynthesis equation?
The light reactions use water as a reactant and produce oxygen. The Calvin cycle uses carbon dioxide as a reactant and produces sugar.
3) Why is the Calvin cycle called a cycle?
The Calvin cycle is a cycle because the starting material, RuBP, is regenerated.
4) What molecule is the direct product of photosynthesis? How is that molecule then used by plant cells?
The direct product of photosynthesis is a small sugar molecule called G3P. With G3P, plant cells can make glucose or any other organic molecule it needs.
Monday, November 10, 2008
Chapter 8.2
- Sunlight is a form of electromagnetic energy
- Electromagnetic energy travels in waves

- Wavelength: distance between two adjacent waves
Visible light: wavelengths you see as different colors - Shorter wavelengths have more energy than longer wavelengths
- Wavelengths shorter than visible light have enough energy to damage organic molecules
- Pigments: chemical compounds that give a substance color
- Light waves are either absorbed, transmitted, or reflected
- Chloroplast pigments absorb blue-violet and red-orange light
- Absorbed light energy converted into chemical energy
- Green light is transmited (passes through leaf) or reflected which is why leaves look green
- In thylakoid membrane, clusters called photosystems
- Photosystems contain few hundred pigment molecules
- Clusters act as a light-gathering panel
- When pigment molecule absorbs light energy, electrons gain energy
- Excited electron is very unstable and passes energy to neighboring molecule
- Excited electron in receiver than passes energy to next pigment molecule
- Energy jumps from molecule to molecule until it reaches reaction center of photosystem
- Reaction center consists of chlorophyll a molecule next to primary electron acceptor
- Primary electron acceptor traps excited electron from chlorophyll a
- Other molecules use energy to make ATP and NADPH

- First photosystem traps light energy and transfers electrons to electron transport chain
- Splits water molecules that releases H+ ions and oxygen as a waste product
- Electron transport chain connecting two photosystems releases energy to make ATP
- Mechanism similar to ATP production in cellular respiration
- H+ ions pumped across thlakoid membrane
- Light-excited electrons from photosynthesis travel down chain
- Second photosystem uses excited electrons and H+ ions to make NADPH
- Calvin cycle uses ATP and NADPH produced by light reactions
Vocabulary
Sunday, November 9, 2008
Chapter 8.1
- Photosynthesis takes place in chloroplast
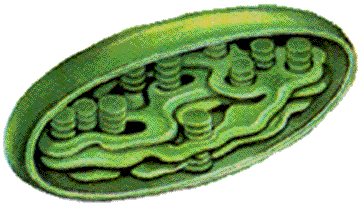
- Green parts of plants carry out photosynthesis
- Chloroplasts are concentrated in the cells of the mesophyll
- The mesophyll is the inner layer of tissue
- Tiny pores called stomata are found on surface of leave
- Carbon dioxide enters and oxygen exits through stomata
- Veins carry water and nutrients from roots to leaves
- Also deliver organic molecules from leaves to other parts
- Chloroplast has inner and outer membrane
- Inner membrane encloses thick fluid called stroma
- Stroma has disk-shaped sacs called thylakoids
- Thylakods organize chemical reactions of photosynthesis
- In photosynthesis, electrons boosted "uphill" by light energy
- Excited electrons plus carbon dioxide and hydrogen ions produce sugar molecules

- 6CO2 + 6H2O --> C6H12O6 + 6O2 = equation for photosynthesis
- Two main stages: light reactions and the Calvin cycle
- Light reactions convert sunlight energy to chemical energy
- Reactions depend on molecules built into thylakoid membranes
- Chlorophyll captures light energy
- Chloroplasts use energy to remove electrons from water
- Splits water into oxygen and hydrogen ions
- Oxygen is "waste product"
- Electrons + hydrogen ions = NADPH
- Light energy also generates ATP
- Overall result: NADPH and ATP
- Calvin cycle makes sugar from atoms in CO2, hydrogen ions, and high-energy electrons carried by NADPH
- Enzymes for Calvin cycle located outside thylakoids and dissolved in stroma
- ATP provides energy to make sugar
- Calvin cycle does not directly require light
- Requires two inputs from light reactions: ATP and NADPH
Tuesday, November 4, 2008
Chapter 7.6 Summary
- When you sprint, lungs and bloodstream can't supply oxygen fast enough to make ATP
Instead, muscle cells use fermentation - Fermentation: making ATP without oxygen
- While cellular respiration still continues, fermentation is main source of ATP

- Fermentation makes ATP from glycolysis
- Yields 2 ATP per glucose molecule
- Fermentation produces lactic acid
- Build up of lactic acid contributes to fatigue after exercise
- Yeast also ferments of sugar and other foods
- Instead of lactic acid, produces alcohol
- Fermentation of fungi and bacteria turn milk into cheese; soybeans into soy sauce; cabbage into sauerkraut
Concept Check 7.6
1) How is fermentation different from cellular respiration?
Fermentation produces ATP without oxygen and only produces 2 ATP per glucose molecule.
2) Describe one example of how fermentation in microorganisms produces human foods
The fermentation of fungi and bacteria is how milk is turned into cheese.
3) What is the waste product of fermentation in your muscle cells?
The waste product of fermentation in our muscle cells is lactic acid.
Chapter 7.5 Summary

- Mitochondria's structure key for cellular respiration
- Inner membrane is highly folded to give more room and maximize ATP production
- Metabolism: all a cell's chemical processes
- Cellular respiration consists of a series of reactions - referred to as a metabolic pathway
- Enzymes catalyze each reaction
- Three main stages of cellular respiration: glycolosis, Krebs cycle, electron transport chain
- Glycolysis: breaking down a glucose molecule
- Takes place in the cytoplasm outside the mitochondria
- Glucose is split in two, each molecule with three carbons and one phosphate group
- Each 3-carb molecule transfers electrons and H ions to NAD+
- NAD+ --> 2 electrons + H ion --> NADH
- Four new ATP molecules produced - net gain of 2 ATP molecules
- 3-carb molecule become pyruvic acid
- Krebs cycle takes place within fluid matrix of inner membrane
- Finishes breaking down pyruvic acid into carbon dioxide
- Glycolysis produces 2 pyruvic acid molecules
- Pyruvic acid loses one CO2 molecule and becomes acetyl CoA
- Acetyl CoA enters Krebs cycle and joins a four-carbon acceptor molecule
- Reactions produce 2 CO2 molecules and 2 ATP (one per acetyl CoA)
- NADH and FADH2 trap most of energy
- At the end, the four-carbon acceptor is regenarated and cycle continues
- Electron Transport Chain occurs in inner membranes of mitochondria
- Two parts: electron transport and ATP production by ATP synthase
- NADH transfers electrons from glucose to electron transport chain
- Electron is moved from carrier to carrier and pulled by oxygen at end of chain
- Each transfer releases a small amount of energy
- This energy pumps H ions across the membrane from less concentrated to more concentrated
- This stores potential energy like a dam holding back water
- ATP synthase: protein structures that create ATP from ADP
- Hydrogen ions are rushed back through the ATP synthase
- ATP synthase uses hte energy to convert ADP to ATP
- Generates up to 34 ATP per glucose molecule
- ATP production requires oxygen
Concept Check 7.5
1) How is the mitochondrion's structure suited to its function?
A mitochondrion's inner membrane has many folds in it so that there are more sites for reactions to occur and maximizes ATP production.
2) Identify the three stages of cellular respiration, where in the cell each takes place, and how many ATP molecules it produces.
First, glycolysis takes place in the cytoplasm and produces two ATP molecules. The Krebs cycle takes place in the inner membranes of the mitochondria and produces two more ATP molecules. The electron transport chain and ATP synthase occur further into the mitochondria and produce up to 34 ATP molecules.
3) Summarize the use and production of ATP in one cycle of cellular respiration.
The production of ATP only requires two ATP molecules as an initial energy investment however it produces up to 38 ATP molecules. So you have a net gain of 36 ATP molecules per cycle.
Sunday, November 2, 2008
Chapter 7.4 Summary
- Cellular respiration is aerobic (requires oxygen)
- Cell takes in oxygen and releases carbon dioxide
- Glucose is common fuel for cellular respiration
- Glucose + 6 Oxygen ---> 6 Carbon Dioxide + 6 Water + ~38 ATP
- When an electron "falls" towards the nucleus potential energy is released
- Oxygen attracts electrons strongly
- Carbon and hydrogen have a much weaker pull
- A sugar molecule has several carbon-hydrogen bonds
- During cellular respiration the bonds are replaced with hydrogen-oxygen and carbon-oxygen bonds
- As electrons fall towards oxygen energy is released
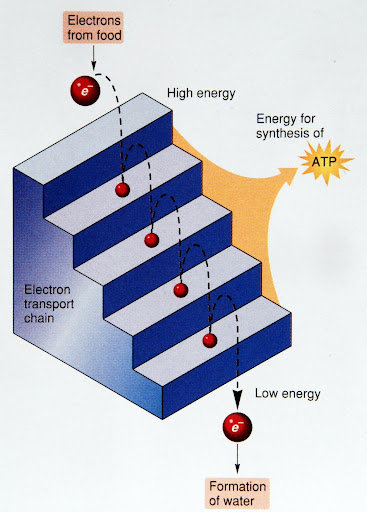 Cellular respiration breaks down glucose in several steps
Cellular respiration breaks down glucose in several steps- Electron carriers accept many high-energy electrons from the glucose
- Electron transport: Electron carriers passing the electrons to the next carrier
- At the end oxygen grabs the electron from the last carrier
- Binds with hydrogen ions to form water
- Between each trasnfer a little energy is released
Chapter 7.3 Summary
 ATP provides energy for cellular work
ATP provides energy for cellular work- ATP: adenosine triphosphate
- adenosine: nitrogen-containing compound adenine + five carbon sugar called ribose
- triphosphate: a "tail" with three phosphate groups
- triphosphate is the source of energy used for most work
- Each phosphate group is negatively charged and repel each other
- ATP tail is like a spring that releases its potential energy when relaxed
- In a chemical reaction one or both phosphate bonds are broken
- Usually only one is resulting in adenosine diphosphate (ADP)
- Three main types of work: chemical work, mechanical work, and transport work
- Chemical work: building large molecules such as proteins
- Mechanical work: contraction of a muscle
- Transport work: pumping solutes across a cellular membrane
- ATP continuously turned into ADP
- ATP can be restored by adding a phosophate group to ADP
- Energy comes from organic molecules in food
Chapter 7.2 Summary
- Energy is the ability to perform work

- Kinetic energy: energy of motion
- Not possible to destroy or create energy
- Energy can be converted from one form to another
- Potential energy: energy stored due to an object's position or arrangement
- Thermal energy: random molecular motion
- Chemical energy: energy from organic compounds
- It is the potential to perform work due to the arrangement of atoms within molecules
- Org. molecules such as carbs, fats, and proteins are rich in chemical energy
- Complex molecules are broken into smaller molecules with less chemical energy than the original substance
- Oxygen + Glucose => Water + Carbon Dioxide
- 40% of energy produced is used
- 60% is lost in the form of heat
- Lost heat helps you maintain body temperature
- Calorie: amount of energy needed to raise the temperature of 1 gram of water by 1 degree Celsius

- Usually measured in kilocalories
- 1 Kilocalorie (kcal) = 1000 calories
- Cells use enzymes to break down organic molecules
Chapter 7.1 Summary
- All organisms need food

- Autotrophs: make their own food by converting inorganic molecules into organic molecules
- Photosynthesis: using the sun's energy to convert water and carbon dioxide into sugars
- Producers = autotrophs
- Heterotrophs: obtain food by eating
 producers or other consumers
producers or other consumers - Consumers = heterotrophs
- Harvest energy from food through cellular respiration
- Cellular respiration: a chemical process that converts chemical energy into ATP using oxygen
- ATP is the main energy supply for plants and animals
- Photosynthesis and cellular respiration recycle water, carbon dioxide, oxygen, and organic compounds like glucose
- Photosynthesis uses water and carbon dioxide to produce glucose and oxygen
- Cellular respiration uses oxygen to produce carbon dioxide and water

Tuesday, September 9, 2008
Chapter 5 Review (pg 106-107)
c. water
2. Which of the following terms includes all the other terms on this list?
a. polysaccharide
3. Which term is most appropriate to describe a molecule that dissolves easily in water?
c. hydrophilic
4. Cholesterol is an example of what kind of molecule?
b. lipid
5. The 20 amino acids vary only in their
b. side groups
6. A specific reactant an enzyme acts upon is called the
d. substrate
7. An enzyme does which of the following?
b. lowers the activation energy of a reaction
8. Besides satisfying your hunger, why else might you consume a big bowl of pasta the night before a race?
It will fill you up with carbohydrates that can be broken down for energy during the race.
9. How are glucose, sucrose, and starch related?
They are all kinds of sugar.
10. What are steroids? Describe two functions they have in cells.
Steroids are special lipids that travel arounnd the body as chemical signals. Estrogen and testosterone work like this and are major components for the male and female appearances. Cholesterol is a steroid found in cell membranes from where other steroids are made.
11. How are polypeptides related to proteins?
Proteins consist of amino acids linked in large chains called polypeptides.
12. How does denaturation affect the ability of a protein to function?
After a protein is unraveled in a process called denaturation, it is no longer able to function properly.
14. Analyzing Diagrams The reaction below shows two amino acids joining together.
a. One product of this reaction is represented by a question mark. Which molecule is it?
water
b. What is this kind of reaction called? Explain.
This is called a dehydration reaction because two hydrogen molecules and an oxygen molecule were removed so the two amino acids could bond. The left over molecules make up water.
c. If an amino acid were added to this chain, at what two places could it attach?
They could attach either to the H on one side or the OH on the other side.
15. Analyzing Graphs Use the graph to answer the questions below.
a. At which temperature does enzyme A perform best? Enzyme B?
Enzyme A performs best around 38 degrees Celsius. Enzyme B performs best around 78 degrees Celsius.
b. Knowing that one of these enzymes is found in humans and the other in thermophilic (heat-loving) bacteria, hypothesize which enzyme came from which organism.
Enzyme A is probably found in humans while enzyme B is probably found in thermophilic bacteria.
c. Propose a hypothesis that explains why the rate of the reaction catalyzed by enzyme A slows down at temperatures above 40°C.
After 40 degrees Celsius, it's hot enough for reactions to take place on their own without the help of an enzyme.
Wednesday, September 3, 2008
Chapter 5.5 Summary
- Enzymes and Activation Energy
- Activation energy: energy that activates the reactants to start a chemical reaction
- Catalysts: Compounds that speed up chemical reactions
- Enzymes: Proteins that are the main catalysts for reactions in organisms
- Lower energy requirement barrier so reactions can occur at normal temperatures
- Each enzyme catalyzes a specific reaction
- How Enzymes Work
- Shape of enzyme fits only particular reactant molecules
- Substrate: Specific reactant acted on by an enzyme

- Active Site: The region of the enzyme that the substrate fits into
- Functional groups of substrate are placed in positions to catalyze reaction
- Enzymes also lower activation energy by accepting two substrates into adjacent sites
- Holding reactants together makes them react easier
- Environment effects enzymes' functioning
Concept Check 5.5
1) Explain the role of activation energy in a reaction. How does an enzyme affect activation energy?
Activation energy is the amount of energy needed to start a chemical reaction. Heat is a common trigger, however heating up a cell could destroy its delicate structures. Enzymes are special proteins that lower the energy required so reactons can occur at a normal temperature.
2) Describe how a substrate interacts with an enzyme.
A substrate enters the enzyme's active site, positioning its functional groups in certain places to catalyze the reaction. The interaction between the substrate and enyzme lowers the activation energy so the chemical reaction can proceed.
Tuesday, September 2, 2008
Chapter 5.4 Summary
Proteins perform most functions in cells
The Functions of Proteins
Protein: A polymer constructed from a set of just 20 kinds of monomers called amino acids
Responsible for day to day functions of organisms
Eg. Make up muscles, defend body from harmful microorganisms, act as signals, control chemical reactions in cells
Amino Acids
Amino acid: A monomer consisting of a central carbon atom bonded to four partners
3 of the bonded partners are always the same: a hydrogen atom, carboxyl group, and amino group
Difference is the "side group" - responsible for chemical properties of each acid
Building a Protein 
Polypeptide: Amino acids linked together to create proteins
Formed by dehydration reaction between amino group and carboxyl group
Variety of proteins because of different amino acids in different orders
Polypeptides at least 100 amino acids in lenghth
Protein Shape
Polypeptides precisely twisted, folded, coiled into unique shape
Sequence of amino acids is important 
Influenced by environment (usually aqueous)
Hydrophilic amino acids - towards outside edges of protein
Hydrophobic amino acids - towards center of protein
Denaturation: When a change in the environment causes a protein to unravel
Denatured protein loses ability to work properly
1) Give at least two examples of proteins you can "see" in the world around you. What are their functions?
Two proteins you can see in the world around you are ones that form structures such as hair or fur, and make up the muscles of an organism.
2) Relate amino acids, polypeptides, and proteins
Proteins are polymers constructed from amino acids, which are monomers with a central carbon atom, that are linked together into a chain called a polypeptides. Most polypeptide chains are around 100 amino acids in length.
3) Explain how heat can destroy a protein.
Heat causes a process called denaturation in a protein which is when the protein unravels, preventing it from functioning properly. The forces that keep the protein in its folded shape are weak bonds between the side groups or a side group and water. Hot molecules collide with enough to force to overcome these weak attractions.
4) Which parts of an amino acid's structure are the same in all amino acids? Which part is unique?
All amino acids share the same three partners bonded to the central carbon atom: a hydrogen atom, a carboxyl group, and an amino group. The part that differentiates them is the side group which is the fourth partner. The side group determines each proteins properties.
Chapter 5.3 Summary
- Characteristics of Lipids
- Lipids: water-avoiding compounds, eg. oil
- Hydrophobic: water-avoiding molecules
- Lipids are boundaries for containing aqueous contents
- Fats store energy in your body
- Fats
- Fat: A three-carbon backbone called glycerol attached to three fatty acids with long hydrocarbon chains
- Fat cushions organs and provides insulation

- Saturated fat: Fat where all three acid chains contain the maximum number hydrogen atoms (All carbons are single bonded)
- Most animal fats are saturated - solid at room temp
- Unsaturated fat: Less than max number of hydrogen because some carbons are double-bonded
- Fats in fruits, vegetable, and fish are usually unsaturated
- Saturated fat can promote buildup of plaque - lipid-containing deposits - which contributes to heart disease
- Steroids
- Steroid: A lipid whose carbon skeleton forms four fused rings

- Different from fats
- Steroids circulate in your body as chemical signals
- Cholesterol: Essential steroid found in cell membranes
- A starting point for producing other steroids
Chapter 5.2 Summary
- Sugars:
- Carbohydrate: an organic compound made up of sugar molecules
- Sugar formula: 1 carbon, 2 hydrogen, 1 oxygen
- Core of sugar molecules are carbon skeletons with a ring shape
- Monosaccharides: Simple sugars containing one sugar unit
- Examples: glucose, fructose, galactose:
- Sugar molecules are main fuel supply for cellular work
- Cells break down glucose to extract energy
- Dissacharides: Two monosaccharides linked together
- Most common dissacharide is sucrose
- Polysaccharides:
- Polysaccharides: Long polymer chains made up of simple sugar monomers
- Starch: A polysaccharide found in plant cells
- Starch chains are sugar stockpiles
- Starch is broken down for glucose
- Glycogen: The polysaccharide animal cells use to store excess sugar
- Glycogen is usually stores as granules in liver and muscle cells
- Cellulose: A polysaccharide in plants used as a building material
- It protects and stiffens plants
- Humans cannot digest cellulose/fiber
- Most carbs are hydrophilic because of hydroxyl groups in sugar unites
- Monosaccharides and dissacharides dossolve easily in water
- Larger carbs (starch, cellulose) can't be dissolved but are still hydrophilic

starch:
Concept Check 5.2
1) Explain the difference between a monosaccharide and a dissacharide. Give an example.
Monosaccharides, such as glucose, consist of only one sugar unit while dissacharides, such as sucrose, consist of two sugar units bonded together
2) Compare and contrast starch, glycogen, and cellulose.
Starch, glycogen, and cellulose are all polysaccharides. Starch and glycogen are used to store energy in cells. Starch however is only found in plant cells, while glycogen is found in animal cells. Cellulose is a polysaccharide found in plants that protects the cells and stiffens the plant to prevent it from flopping over.
3) How do animals store excess glucose molecules?
Animals store glucose in the form of a polysaccharide called glycogen. glycogen is usually stored as granules in the liver and muscle cells. When the energy is needed, the glycogen granules are broken down to release the glucose.
Chapter 5.1 Summary
- Carbon Skeletons and Functional Groups
- Carbon can form up to 4 bonds with other atoms
- Organic molecules: carbon-based molecules
- Inorganic molecules: non-carbon-based molecules, eg. water/oxygen/ammonia
- Hydrocarbons: organic molecules composed of only carbon and hydrogen atoms
- Two other atoms often in organic molecules are oxygen and nitrogen
- Functional group: A group of atoms within a molecule that interact in predictable ways with other molecules
- Carbon skeleton and functional groups determine properties of org. molecules
- Hydrophilic: attract water molecules
- Monomers and Polymers
- Biomolecules composed of hundreds or millions of atoms
- Monomers: Similar, smaller molecular units
- Polymers: Long chains of monomers linked together
- May be straight chains, branching chains, or chains folding back on themselves
- Thousands of polymers built by fewer than 50 types of monomers
- Four categories of life's main molecules: carbohydrates, lipids, proteins, nucleic acids
- Building and Breaking Polymers
- Dehydration Reaction: When a water molecule is released when a monomer is added
- Polymers must be broken down
- Food is broken down to make monomers available
- Either break down monomers for energy to build new polymers
- Hydrolysis Reaction: When water is added to break bonds between monomers
Concept Check 5.1
1) Draw a molecule that has a three-carbon skeleton and a hydroxyl group on the middle carbon.

2) Explain the connection between monomers and polymers.
Monomers are the small molecular units that are linked together to create polymer.
3) What molecule is released during the construction of a polymer? What is this reaction called?
A water molecule is released during the construction of a polymer in a reaction called a dehydration reaction.
4) Draw at least three ways in which five carbon atoms could be joined to make different carbon skeletons.
Friday, August 29, 2008
ME.
Long long ago
In the scary States of the United America
I was born in the Apple of Bigness!!! (New York)
On december 13 in Teach Medical Center.
When I had existed for 3 years, I was relocated to Zhong Hua Ren Min Gong He Guo.
In the city of Guangzhou.
Then, in the summer of my fifth year of living
I moved to the Golden State of In-n-Out and Arnold Swarzhenagger.....however you spell his name
I stayed there until the end of the grade 7 in middle school.
Then, I returned to Maoland.
I enrolled in the International Guangzhou School of the States of United America for the ochoest grade.
And that's where I am today :)
My hobbies of liking are:
Drumsticking
Guitarofelectricitypicking
Listeningtowethekingsuntilivememorizedalltheirsongsing
Eatingmarshmallowsing
Photoshopfacesintootherfacesing
Youtubeing
Boardonsnowing
Throworangeballsintoahooping
Kickblackandwhiteballsintoaneting
and other fun stuff
:D